The six principles of patient partnerships
The Patients Association have published the ‘six key principles of patient partnership’, a concept which [...]
05
Dec
Dec
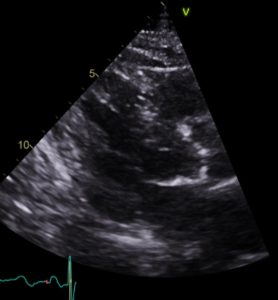
Non-invasive high energy ultrasound for calcific aortic stenosis: game-changer or damp squib?
Comment on: Messas et al. Treatment of severe symptomatic aortic valve stenosis using non-invasive ultrasound [...]
21
Nov
Nov
New ESC guidelines on management of endocarditis published
2023 ESC Guidelines for the management of endocarditis | European Heart Journal | Oxford Academic [...]
23
Oct
Oct
BHVS on X
We were based for the day in the beautiful Dorchester Library on the first floor [...]
16
Oct
Oct
Inequalities in access to aortic valve replacement laid bare
A new publication co-authored by past-presidents of BHVS highlights the inequalities facing women, ethnic minorities [...]
11
Oct
Oct
Statements
Statements issued by the BHVS Statements issued by the BHVS, sometimes as a Joint Statement [...]
22
May
May